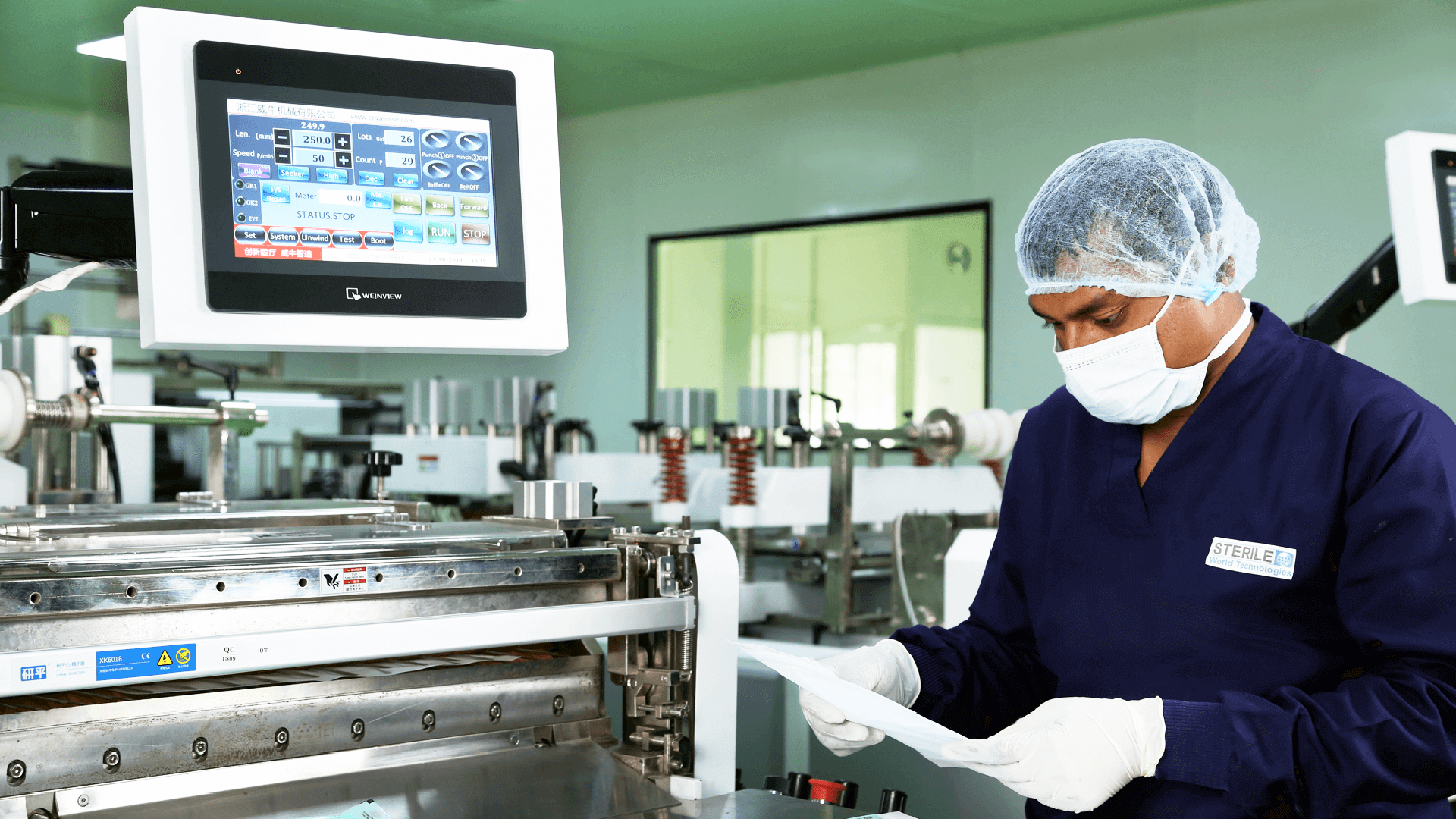
Medical Device Contract Manufacturing
Contract Cleaning
Cleaning And Rinsing
BY : STERILE WORLD TECHNOLOGIESHow a medical device is machined will dictate the most effective cleaning method. Typically, this cleaning method will entail rinsing, passivation or ultrasonic cleaning depending on the level of impurities to be removed as well as it’s
cleanliness requirements. SWT offers following forms of medical device cleaning and rinsing:
- Cleaning with & without detergents
- Ultrasonic cleaning and drying
- Rinsing & disinfecting
All water used in the rinsing and cleaning process meets the requirements of European Pharmacopoeia.
Final Decontamination – Washing & Disinfecting
BY : STERILE WORLD TECHNOLOGIESSWT specialises in fully automated, microprocessor-controlled mechanical processes, specifically designed for medical device cleaning. Our cleaning processes are designed to handle moisture and temperature-stable devices prior to further processing – for example blister packaging and sterilisation.
Each rinsing cycle is individually designed and validated to meet the requirements of our Customers’ devices and, prior to processing, the SWT Group experts will advise on the most appropriate rinsing and cleaning cycle for their devices.

Ultrasonic Cleaning And Drying
BY : STERILE WORLD TECHNOLOGIESUltrasonic cleaning is a process that uses ultrasound and, when needed, an appropriate cleaning solvent to clean devices. Each cleansing cycling is carefully designed by the SWT Group experts to ensure effective removal of impurities.
Packaging Features
A specially designed medical grade paperwhich correspond to the requirements of DIN EN ISO 11607 and EN 868.
Manufactured using 60 & 70 g/ml medical heavy-duty paper and medical grade film laminate (Petand Poly) CPPfilm.
- Printing of labels & indicators can be done according to the customer's requirements.
- Offers easy peeling without delamination
- Products can be customized as per special sizes and special formats.
- Special lengths upto 1,500 mm on Inquiry are available.
Customer Benefits
The packaging can maintains the sterility up to 180 days*
Best in peel ability
The STERILITY range offers one of the best peel ability performances on the market.
Wide range
STERILITY range exists in flat pouches and reels, gusseted pouches and reels, and self-seal pouches.
Safe for surgery timing
No curling on opening = no loss of time due to “contamination at opening" suspicions.
